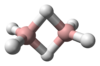Chemistry:Tetraoxidane
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetraoxidane
| |||
| Other names
Hydroxyperoxide, dihydrogen tetroxide, diperoxide, bisperoxide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| H2O4 | |||
| Molar mass | 66.012 g·mol−1 | ||
| Density | 1.8±0.1 g/cm3 | ||
| Related compounds | |||
Related compounds
|
Pentaoxidane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tetraoxidane is an inorganic compound of hydrogen and oxygen with the chemical formula H2O4.[1][2][3] This is one of the unstable hydrogen polyoxides.[4]
Synthesis
The compound is prepared by a chemical reaction between hydroperoxyl radicals (HO
2•) at low temperatures:[5][6]
- [math]\displaystyle{ \mathrm{2HO_2^\bullet \xrightarrow{} 2H_2O_4} }[/math]
Physical properties
This is the fourth member of the polyoxidanes. The first three are water [(mon)oxidane], hydrogen peroxide (dioxidane), and trioxidane. Tetroxidane is more unstable than the previous compounds. The term "tetraoxidane" extends beyond the parent compound to several daughter compounds of the general formula R2O4, where R can be hydrogen, halogen atoms, or various inorganic and organic monovalent radicals. The two Rs together can be replaced by a divalent radical, so heterocyclic tetroxidanes also exist.[7]
Ionization
Tetroxidane autoionizes when in liquid form:
- [math]\displaystyle{ \mathrm{H_2O_4 \rightleftarrows H^+ + HO_4^-} }[/math]
- [math]\displaystyle{ \mathrm{2H_2O_4 \rightleftarrows H_3O_4^+ + HO_4^-} }[/math]
References
- ↑ Mckay, Daniel J.; Wright, James S. (1 February 1998). "How Long Can You Make an Oxygen Chain?" (in en). Journal of the American Chemical Society 120 (5): 1003–1013. doi:10.1021/ja971534b. ISSN 0002-7863. https://pubs.acs.org/doi/pdf/10.1021/ja971534b. Retrieved 16 May 2023.
- ↑ "hydroxyperoxide". ChemScr. https://www.chemsrc.com/en/cas/29683-94-1_1277401.html.
- ↑ (in en) The Chemistry of Peroxides, Volume 3. John Wiley & Sons. 20 April 2015. p. 198. ISBN 978-1-118-41271-8. https://books.google.com/books?id=hX3mBgAAQBAJ&dq=tetraoxidane&pg=PA198. Retrieved 15 May 2023.
- ↑ "Selected ATcT [1, 2 enthalpy of formation based on version 1.122 of the Thermochemical Network [3]"]. atct.anl.gov. https://atct.anl.gov/Thermochemical%20Data/version%201.122/species/?species_number=1035.
- ↑ Levanov, Alexander V.; Sakharov, Dmitri V.; Dashkova, Anna V.; Antipenko, Ewald E.; Lunin, Valeri V. (2011). "Synthesis of Hydrogen Polyoxides H2O4 and H2O3 and Their Characterization by Raman Spectroscopy". European Journal of Inorganic Chemistry 2011 (33): 5144–5150. doi:10.1002/ejic.201100767.
- ↑ Möller, Detlev (19 February 2019) (in en). Fundamentals and Processes. Walter de Gruyter GmbH & Co KG. p. 276. ISBN 978-3-11-056126-5. https://books.google.com/books?id=MOaTDwAAQBAJ&dq=h2o4+tetraoxidane&pg=PA276. Retrieved 15 May 2023.
- ↑ Curutchet, Antton; Colinet, Pauline; Michel, Carine; Steinmann, Stephan N.; Le Bahers, Tangui (2020). "Two-sites are better than one: revisiting the OER mechanism on CoOOH by DFT with electrode polarization". Physical Chemistry Chemical Physics 22 (13): 9. doi:10.1039/D0CP00281J. Bibcode: 2020PCCP...22.7031C. https://hal.science/hal-02519600v1/file/CoOOH-Main-PCCP-2.pdf. Retrieved 15 May 2023.
 |