Chemistry:Tetrahydrocannabiphorol
 | |
 | |
| Clinical data | |
|---|---|
| Other names | (-)-Trans-Δ9-tetrahydrocannabiphorol Δ9-THCP (C7)-Δ9-THC THC-Heptyl |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C23H34O2 |
| Molar mass | 342.523 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC,[1] but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa.[2][3] It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." Tetrahydrocannabiphorol has a reported binding affinity of 1.2 nM at CB1, approximately 33 times that of Δ9-THC (40 nM at CB1).[4] THCP was studied by Roger Adams as early as 1942.[5]
Isomers
Delta-3-THCP
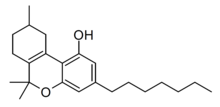
The Δ3/Δ6a(10a) isomer Δ3-THCP was synthesised in 1941, and was found to have around the same potency as Δ3-THC, unlike the hexyl homologue parahexyl which was significantly stronger.[6]
Delta-8-THCP

The Δ8 isomer is also known as a synthetic cannabinoid under the code name JWH-091.[7][8] It's unconfirmed whether or not Δ8-THCP is found naturally in cannabis plants, but likely is due to Δ8-THC itself being a degraded form of Δ9-THC.[9] JWH-091 has approximately double the binding affinity at the CB1 receptor (22 nM ± 3.9 nM) in comparison to Δ9-THC (40.7 nM ± 1.7 nM) or Δ8-THC (44 nM ± 12 nM),[8] but appears significantly lower in vitro than the binding activity of Δ9-THCP (Ki = 1.2 nM)[4]
Natural occurrence in Cannabis
Δ9-THCP occurs naturally in Cannabis, but in small amounts. An analysis on mid to high THC strains ranged approximately from 0.0023% to 0.0136% (w/w) (approximately 0.02–0.13 mg/g) with no correlation with THC percentage, such a strain with 8% THC vs 20% THC both with similar amounts of THCP.[2]
See also
- 1,2-Didehydro-3-oxo-THCO
- CBD-DMH
- Delta-8-THC
- Hexahydrocannabinol
- Hexahydrocannabiphorol
- HU-210
- JWH-138
- Parahexyl
- Perrottetinene
- Tetrahydrocannabivarin
- Tetrahydrocannabutol
- Tetrahydrocannabihexol
- THCP-O-acetate
- O-1871
- DMHP
- Cannabicyclohexanol
References
- ↑ "Identification of hepatic metabolites of n-heptyl-delta-1-tetrahydrocannabinol in the mouse". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 15 (3): 187–197. March 1985. doi:10.3109/00498258509045349. PMID 2992174.
- ↑ 2.0 2.1 "(-)-trans-Δ9-Tetrahydrocannabiphorol Content of Cannabis sativa Inflorescence from Various Chemotypes". Journal of Natural Products 84 (2): 531–536. February 2021. doi:10.1021/acs.jnatprod.0c01034. PMID 33565878.
- ↑ "The novel heptyl phorolic acid cannabinoids content in different Cannabis sativa L. accessions". Talanta 235: 122704. December 2021. doi:10.1016/j.talanta.2021.122704. PMID 34517579.
- ↑ 4.0 4.1 "A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol". Scientific Reports 9 (1): 20335. December 2019. doi:10.1038/s41598-019-56785-1. PMID 31889124.
- ↑ https://pubs.acs.org/doi/abs/10.1021/ja01255a061
- ↑ "Tetrahydrocannabinol Homologs with Marihuana Activity. IX.". Journal of the American Chemical Society 63 (7): 1971–1973. July 1941. doi:10.1021/ja01852a052.
- ↑ "Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists". The Journal of Pharmacology and Experimental Therapeutics 290 (3): 1065–1079. September 1999. PMID 10454479. https://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=10454479.
- ↑ 8.0 8.1 "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry 8: 17–39. 2016. doi:10.4137/PMC.S32171. PMID 27398024.
- ↑ "Chemistry of Cannabis". Comprehensive Natural Products II. 2010. pp. 1033–1084. doi:10.1016/B978-008045382-8.00091-5. ISBN 978-0-08-045382-8. https://books.google.com/books?id=ISBN9780080453828&pg=PA1033.
 |

