Chemistry:1,1'-Diaminoferrocene
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
1,1'-Diaminoferrocene
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C10H12FeN2 | |
| Molar mass | 216.065 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.644 g/cm3[1] |
| Melting point | 183–186 °C (361–367 °F; 456–459 K) |
| Boiling point | decomposition |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
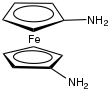
1,1'-Diaminoferrocene is the organoiron compound with the formula Fe(C
5H
4NH
2)
2. It is the simplest diamine derivative of ferrocene. It is a yellow, air-sensitive solid that is soluble in aqueous acid. The 1,1' part of its name refers to the location of the amine groups on separate rings. Compared to the parent ferrocene, the diamine is about 600 mV more reducing.[1]
It can be prepared from the diisocyanate Fe(C
5H
4NCO)
2, which in turn is derived from 1,1'-ferrocenedicarboxylic acid.[2] 1,1'-Diaminoferrocene was originally prepared by hydrogenation of 1,1'-diazidoferrocene ( Fe(C
5H
4N
3)
2).[1]
1,1'-Diaminoferrocene has been incorporated into various diamide and diimine ligands, which form catalysts that exhibit redox switching.[3]
References
- ↑ 1.0 1.1 1.2 Shafir, Alexandr; Power, Maurice P.; Whitener, Glenn D.; Arnold, John (2000). "Synthesis, Structure, and Properties of 1,1'-Diamino- and 1,1'-Diazidoferrocene". Organometallics 19 (19): 3978–3982. doi:10.1021/om0004085.
- ↑ Petrov, Alex R.; Jess, Kristof; Freytag, Matthias; Jones, Peter G.; Tamm, Matthias (2013). "Large-Scale Preparation of 1,1′-Ferrocenedicarboxylic Acid, a Key Compound for the Synthesis of 1,1′-Disubstituted Ferrocene Derivatives". Organometallics 32 (20): 5946–5954. doi:10.1021/om4004972.
- ↑ Wang, Xinke; Thevenon, Arnaud; Brosmer, Jonathan L.; Yu, Insun; Khan, Saeed I.; Mehrkhodavandi, Parisa; Diaconescu, Paula L. (2014). "Redox Control of Group 4 Metal Ring-Opening Polymerization Activity toward l-Lactide and ε-Caprolactone". Journal of the American Chemical Society 136 (32): 11264–11267. doi:10.1021/ja505883u. PMID 25062499. https://escholarship.org/uc/item/5hm8f76w.
 |

